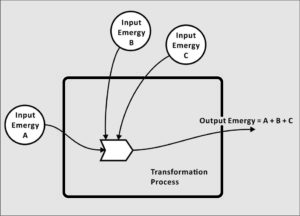
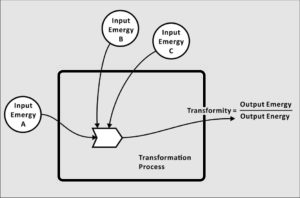
What is Emergy?
Emergy is perhaps the most significant, but least known or understood quantity in the realm of science. It is a quantity derived from the laws of thermodynamics, including the proposed 4th Law of Thermodynamics, the maximum power principle (Lotka, 1922), which was elaborated by Odum as the maximum empower principle (1996).
Emergy is not a physical quantity, per se. By this we mean that there is no place in the universe where you can make a physical measurement that will be equivalent to a quantity of emergy. It is the memory of the available energy used directly and indirectly to produce a service or product. It is the Energy Memory, the emergy.
The available energy of an item is the potential energy within that item that can do work, such as that in a log from a tree that releases a measurable quantity of potential energy by burning the wood, degrading it into a quantity of heat. The available energy in the log can be measured in calories or joules, using the English or metric systems, respectively. The log contains a quantity of available energy that can be measured by the heat released when it is burned.
The emergy of the log, on the other hand, is the available energy that was used-up in past transformations, which were required to create the available energy in the log. Emergy measures then the energy in sunlight and transpiration used in growing the tree, it measures the available energy used to harvest the tree and process it into logs, and other contributions that resulted in the log that lays in your fireplace to be burned. Emergy is the record of the previously used-up available energy that is manifested in an item in the present, in this case, the easily measured available energy in the log.
Comparability
Joules or calories of available energy are not comparable in different objects or services from different systems of production. The available energy in a log is not comparable to the available energy in a paper document like the US Constitution, or in the human metabolic energy of its famous signators. Emergy, by contrast, allows the comparison of flows, products, or services such as these on an equal basis, using the emergy unit, or solar emjoule, sej.
System Self-Organization
And emergy has deeper significance as a thermodynamic quantity that demonstrates the maximization of quality–adjusted available energy flow or empower (emergy per unit time). Maximizing empower flow through a system defines the decision criterion in evolutionary competition between alternative designs.
Transformity and Its Importance
Transformity is a conversion value that transforms energy to emergy. It therefore relates the past use of available energy (emergy) in a production process to the available energy remaining in the item produced. Its units in the metric system are solar emjoules per joule (sej/J). Over time, characteristic transformities come to be associated with known goods or services, and are applied to calculate emergy inputs to other production processes.
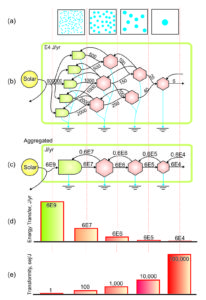
Energy Transformation Hierarchy
Transformity has important implications for emergy theory, because it is used to relate all of the forms of energy in a series of energy transformations to one form of energy (usually solar joules). In a simple chain of energy transformations the energy decreases at each step up the chain (d, diagram), due to losses required by the 2nd Law of Thermodynamics, but the emergy contributed at the base of the chain is the same. As a result the transformity (sej/J) of the remaining available energy increases with each transformation (e, diagram).
Hierarchy Position
When chains of transformations of available energy are combined in a complex network of energy transformations, transformity measures the position of each kind of energy within the universal energy hierarchy (i.e., the network of interconnected energy flows).
Quality
For this reason, transformity is a quantitative measure of the relative quality of everything produced within a system. The importance of transformity is that everyone seeks quality in their choices. People perceive quality, subjectively, and there are partial measures of quality such as, the degree of concentration, e.g., 24 carat gold. But transformity is the only universal objective measure of quality known to science.
Efficiency
For the same or equivalent products, transformity is also a measure of the relative efficiency of production processes. Here the minimum transformity indicates the most efficient production process, because the maximum output (J of a product) is produced per unit of emergy input.